
If you do not know how to dissolve your sample properly, especially how to dissolve hydrophobic peptides, it will often make it difficult for you to carry out the experiment. Sample consumption is inevitable to determine the optimal dissolution conditions of the peptide. If the consumption is too much, the remaining key peptide sample may not be enough to complete your subsequent experiments.
We provide solubility testing service to help you deal with hydrophobic peptides in a more customized way. You will not need to estimate the solubility of the sample through additional experiments, we will provide you with a complete, customized peptide solubility properties report, specifically for you to solve the problem of peptide dissolution properties.
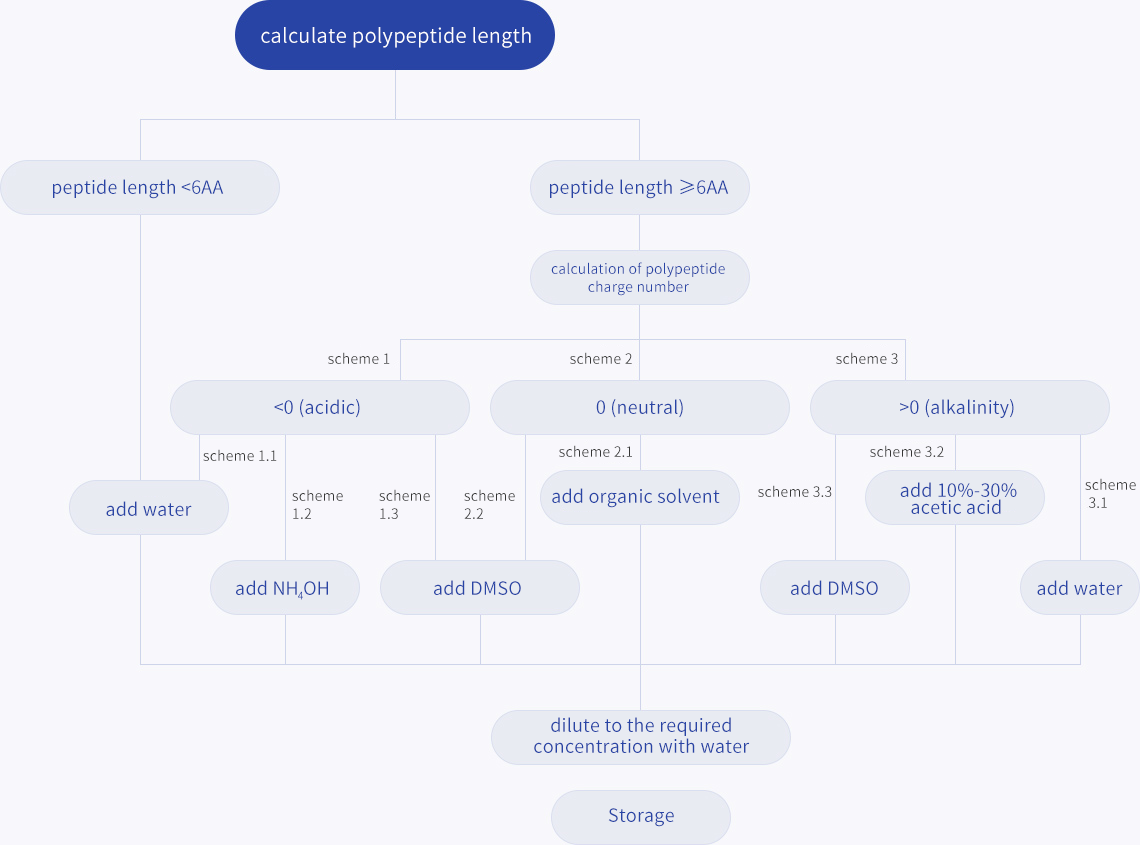
Suitable polypeptide dissolved polypeptide analysis experiment is an important factor for success. Improper or inadequate dissolution of peptides can lead to inaccurate calculation of peptide concentrations and may cause experimental errors or lead to experimental failure.
It is recommended that a small amount of peptide be used first to test the optimal solution. Only when the peptide is completely dissolved can the solution be added and diluted to the final concentration. That is, dissolve first and then add buffer. Note that the peptide is always added to the appropriate solvent to stir, not otherwise.

All products labeled "freeze-dried" must be kept in freezing conditions, preferably at -20 ° C, but most peptides may remain active for several years if kept below -80 ° C.
When using frozen products, the bottle or test tube should be raised to room temperature in a drying oven with fresh desiccant before opening the lid. For products stored at -20°C, the process takes an hour or more, depending on package size. Otherwise, when the bottle is opened, water vapor enters and causes the peptide to condense, reducing its stability. Once opened, should be quickly weighed, and immediately closed to avoid deliquescence, especially hydrophilic peptide should pay more attention to.
Polypeptide solutions are much less stable than dry powders. For best results, follow the following principles:
Repeated freezing and thawing damage the activity of the peptide, so it is recommended to separate into small packaging. Defrost as much as you need and discard the rest after use.
Soluble in PH5-7 sterile buffer solution, stored at -20° C.
Peptides containing Cys, Met, Typ, Glu and Asp are easy to oxidize and should be stored in an oxidant-free environment.
Since bacteria can degrade peptides, they must be filtered for removal before storage.