Staple peptide
Studies have shown that peptides with α-helical structure and high positive charge can cross cell membranes. However, once separated from the parent, the original secondary structure cannot be maintained. The conformational instability leads to the weak binding effect of the protein, while ordinary linear peptides cannot cross the cell membrane and are easily hydrolyzed. After continuous attempts, Verdine et al. developed a novel structure of polypeptide, this polypeptide is called stapler peptide, it is a kind of all-carbon scaffold with α-helix structure of polypeptide, the all-carbon scaffold stable α-helix structure, enhance the interaction of peptide molecules and proteins, and stapler peptide can pass through the cell membrane, not easy to be hydrolyzed, Compared with the previous small molecule drugs and protein drugs, it has higher pharmacological activity.
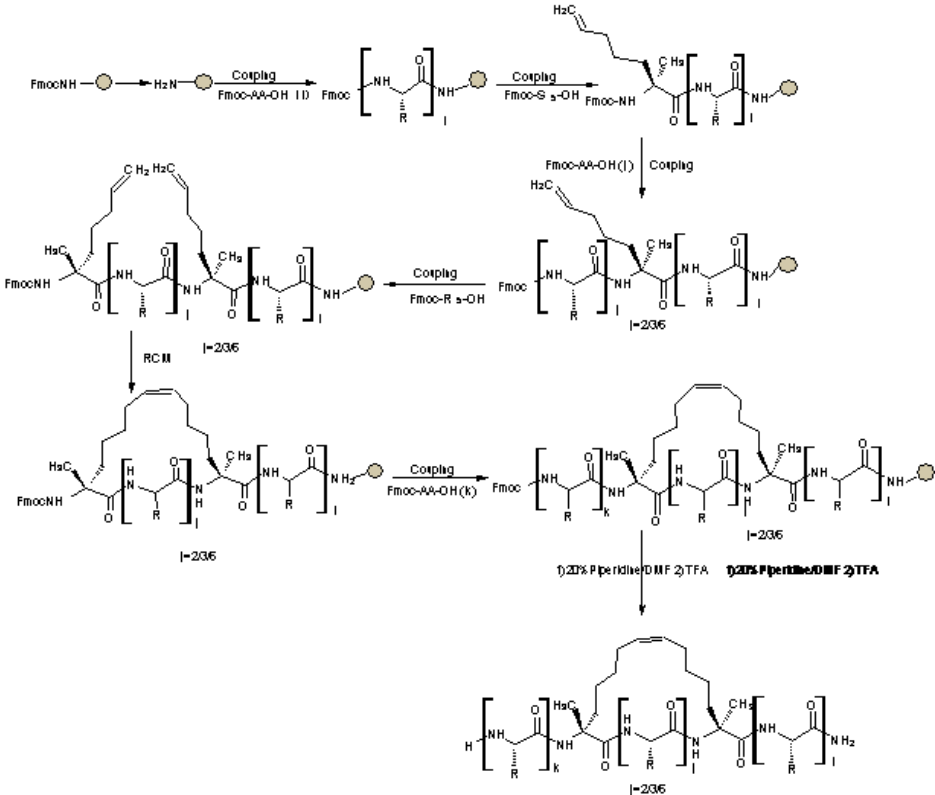
The difference between staple peptide synthesis and common peptide synthesis is that two non-natural amino acids containing α-methyl and α-enyl are introduced into the solid phase synthesis of peptide chain, and then the olefin complex decomposition reaction between the two non-natural amino acids is cyclized to form the whole carbon scaffold with stable α-helical structure, and then the staple peptide is synthesized.
The general synthesis route of stapled peptides is:


 Contact Us
Contact Us
 Return
Return